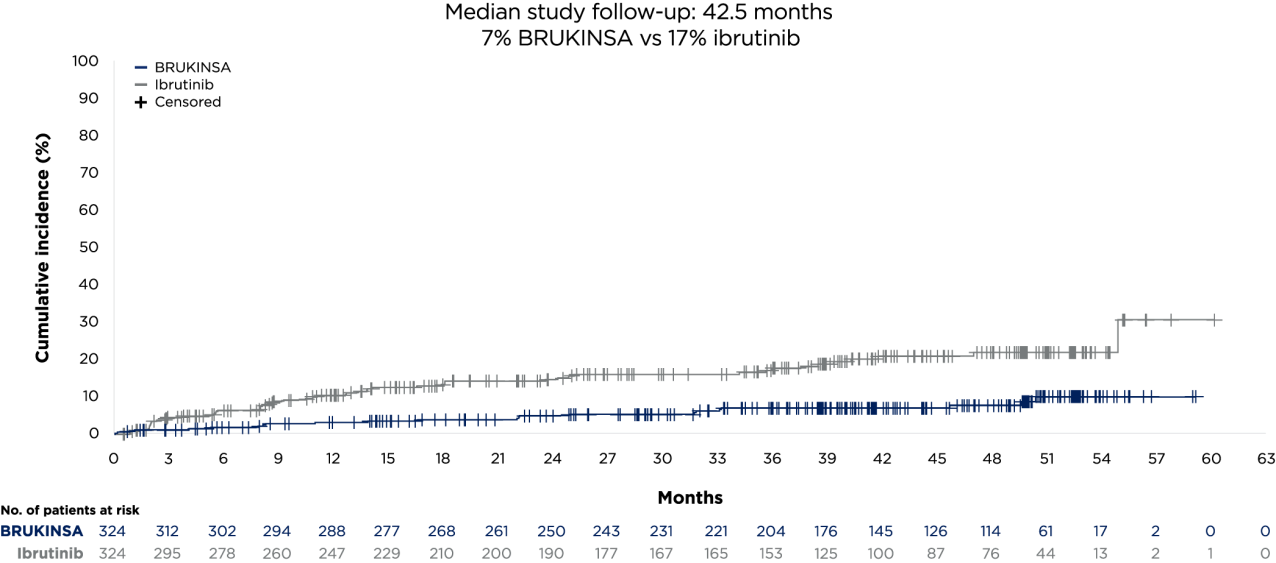
The pivotal ALPINE study (NCT03734016) is a global, randomised, open-label, head-to-head, Phase 3 study for the treatment of patients with R/R CLL/SLL.3,4
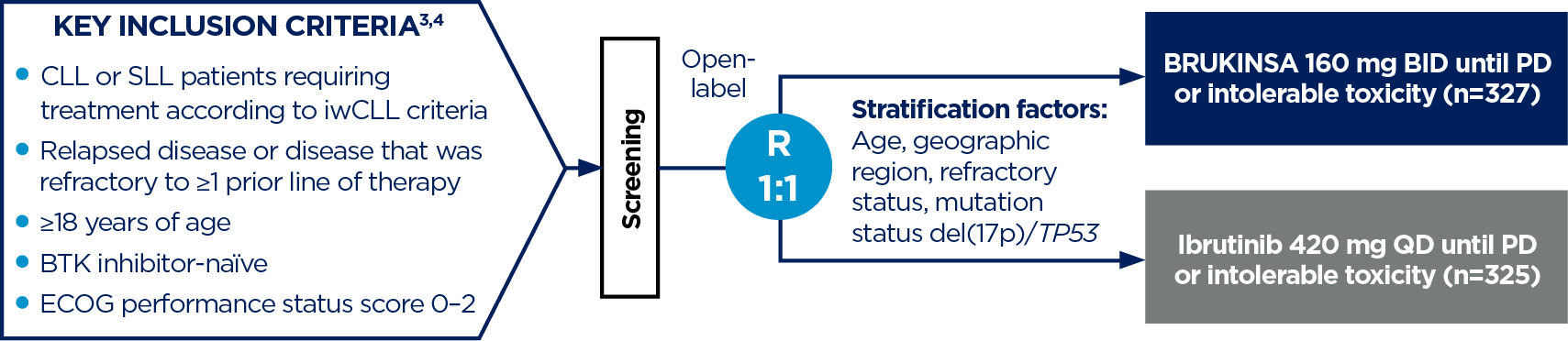

- Primary endpoint: Investigator-assessed ORR (PR + CR)
- Key secondary endpoints: Investigator-assessed PFS, incidence of atrial fibrillation or flutter
- Other secondary endpoints: PR-L or better, DOR, time to treatment failure, OS, safety