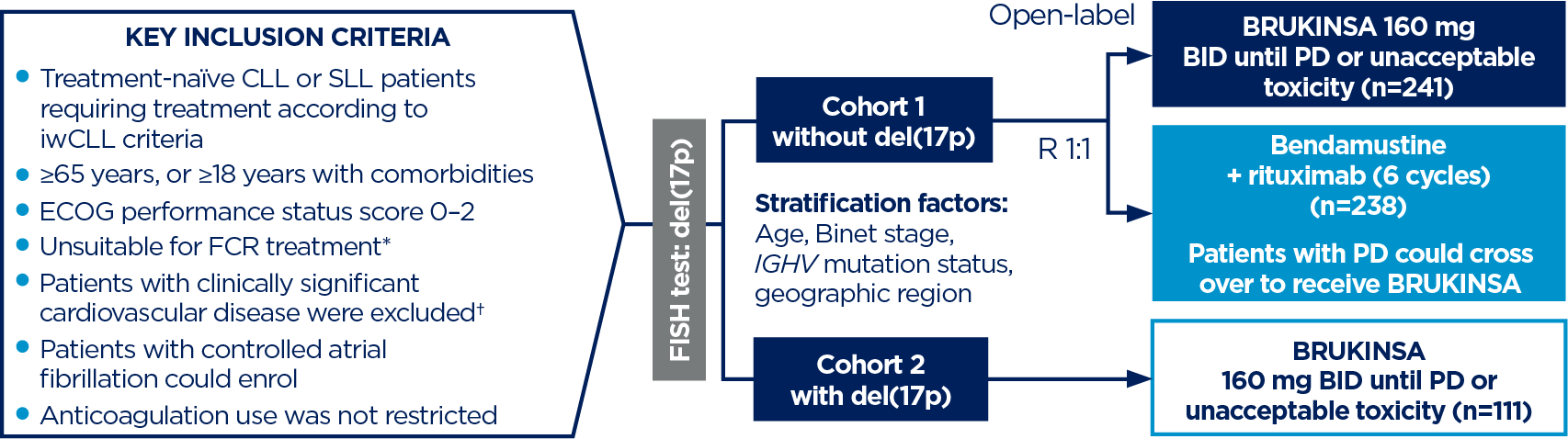
*Defined as ≥65 years, or 18–64 years with one of the following: CIRS score >6, creatinine clearance <70 mL/min, or severe or frequent infections during the course of illness.3
†Such as recent myocardial infarction, unstable angina, severe congestive heart failure, uncontrolled hypertension or uncontrolled arrhythmias.3
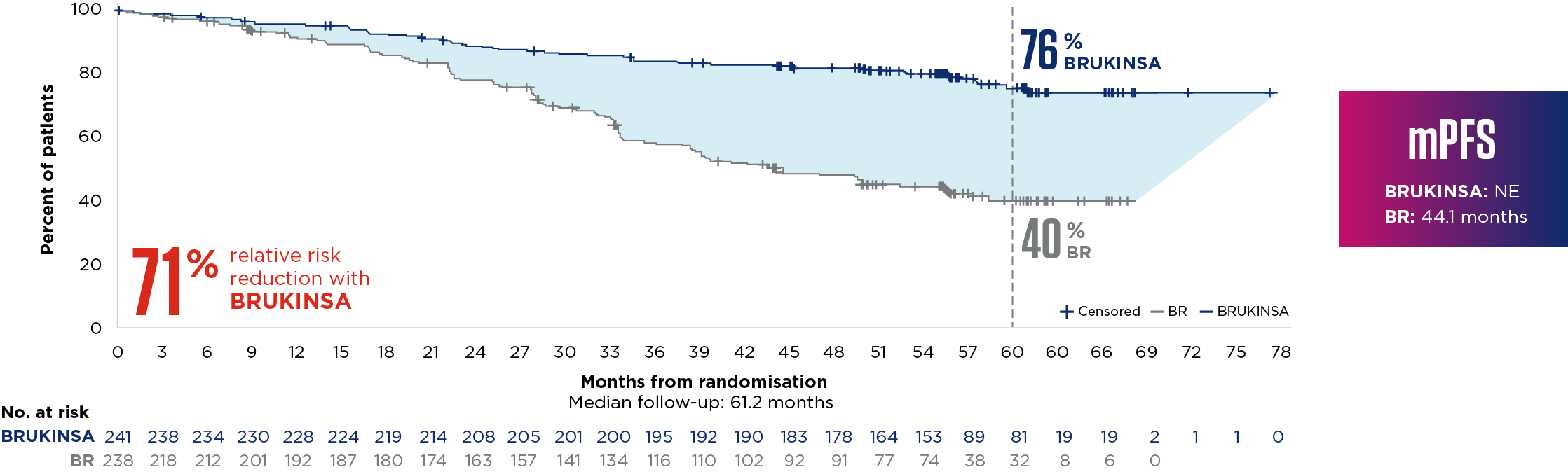
‡Median duration of exposure: Cohort 1 BRUKINSA 60.5 months, BR 5.5/5.6 for bendamustine/rituximab. EAIRs were calculated as the number of patients with a treatment-emergent AE in each category divided by the total time from the first dose date to the first event date, or the exposure time if no event occurred.4
AE, adverse event; BID, twice daily; BR, bendamustine + rituximab; BTK, Bruton’s tyrosine kinase; CI, confidence interval; CIRS, Cumulative Illness Rating Scale; CIT, chemo-immunotherapy; CLL, chronic lymphocytic leukaemia; COVID-19, coronavirus disease 2019; DOR, duration of response; EAIR, exposure-adjusted incidence rate; ECOG, Eastern Cooperative Oncology Group; FCR, fludarabine-cyclophosphamide-rituximab; FISH, fluorescence in situ hybridisation; GI, gastrointestinal; HR, hazard ratio; IGHV, immunoglobulin heavy chain variable region; IRC, independent review committee; iwCLL, International Workshop on CLL; MCL, mantle cell lymphoma; mPFS, median progression-free survival; MZL, marginal zone lymphoma; NS, not stated; ORR, overall response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; QoL, quality of life; R, randomisation; SLL, small lymphocytic lymphoma; TN, treatment-naïve; WM, Waldenström’s macroglobulinaemia.
References:
- BRUKINSA. United Kingdom Summary of Product Characteristics. BeOne Medicines UK Ltd.;
- Tam CS, et al. Blood Cancer J. 2023;13(1):141;
- Tam CS, et al. Lancet Oncol. 2022;23(8):1031–1043;
- Shadman M, et al. J Clin Oncol. 2025;43(7):780–787;
- Tam CS, et al. ASCO Annual Meeting, May 30–June 3, 2025. Abstract #7011;
- Ghia P, et al. Curr Med Res Opin. 2023;39(11):1505–1511;
- Tam CS, et al. Expert Rev Clin Pharmacol. 2021;14(11):1329–1344;
- Brullo C, et al. Int J Mol Sci. 2021;22(14):7641;
- Shadman M, et al. Lancet Haematol. 2023;10(1):e35–e45;
- Guo Y, et al. J Med Chem. 2019;62(17):7923–7940;
- Imbruvica. Summary of Product Characteristics. Pharmacyclics LLC, Janssen Biotech, Inc.;
- Calquence. Summary of Product Characteristics. AstraZeneca Pharmaceuticals LP.
The study is registered with ClinicalTrials.gov: NCT03336333