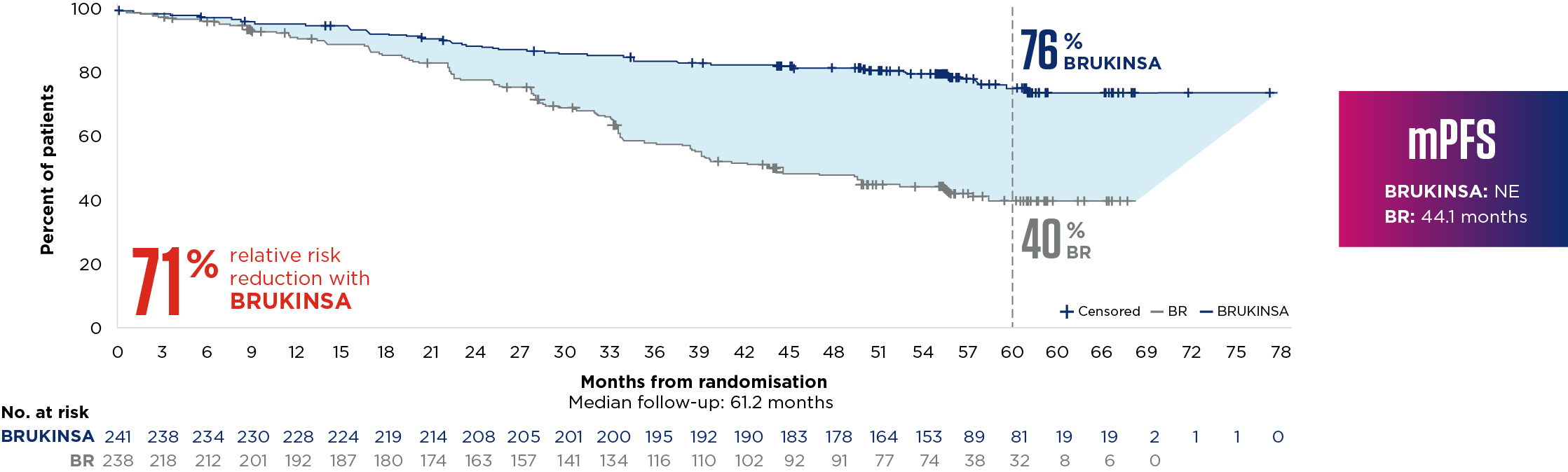
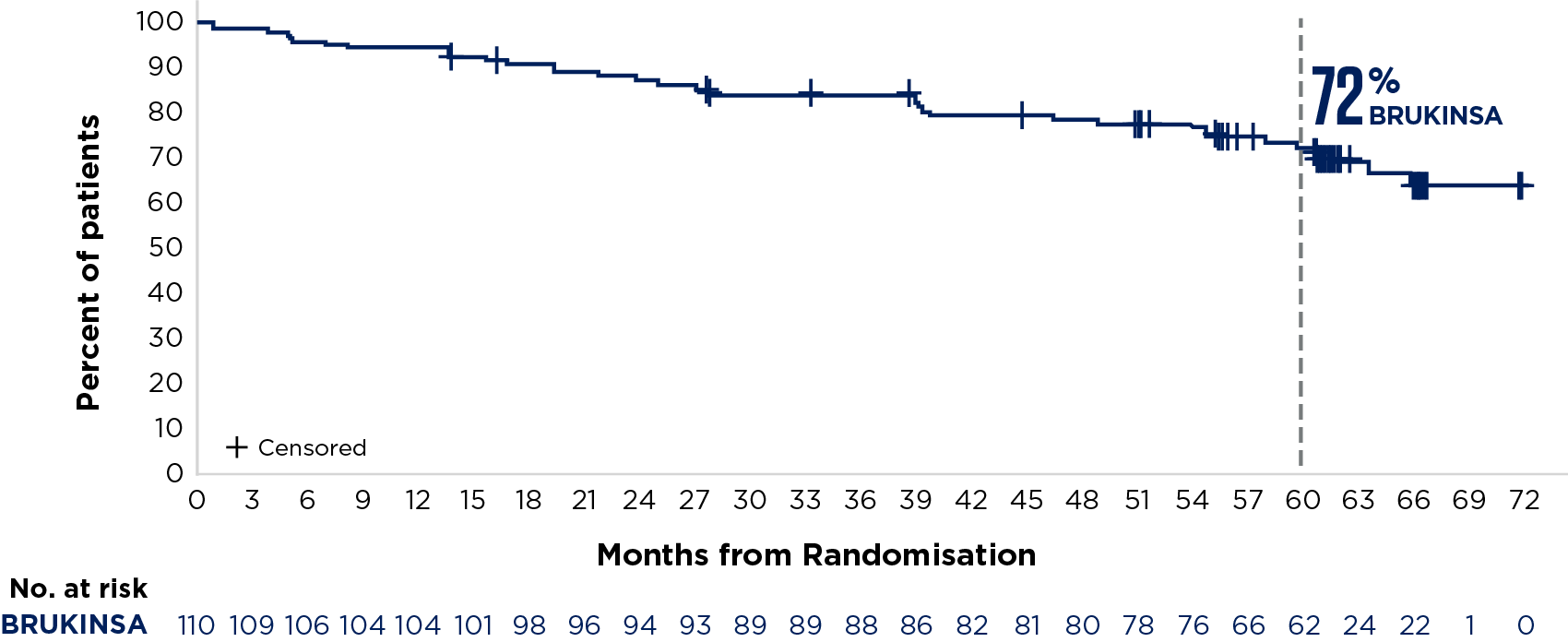
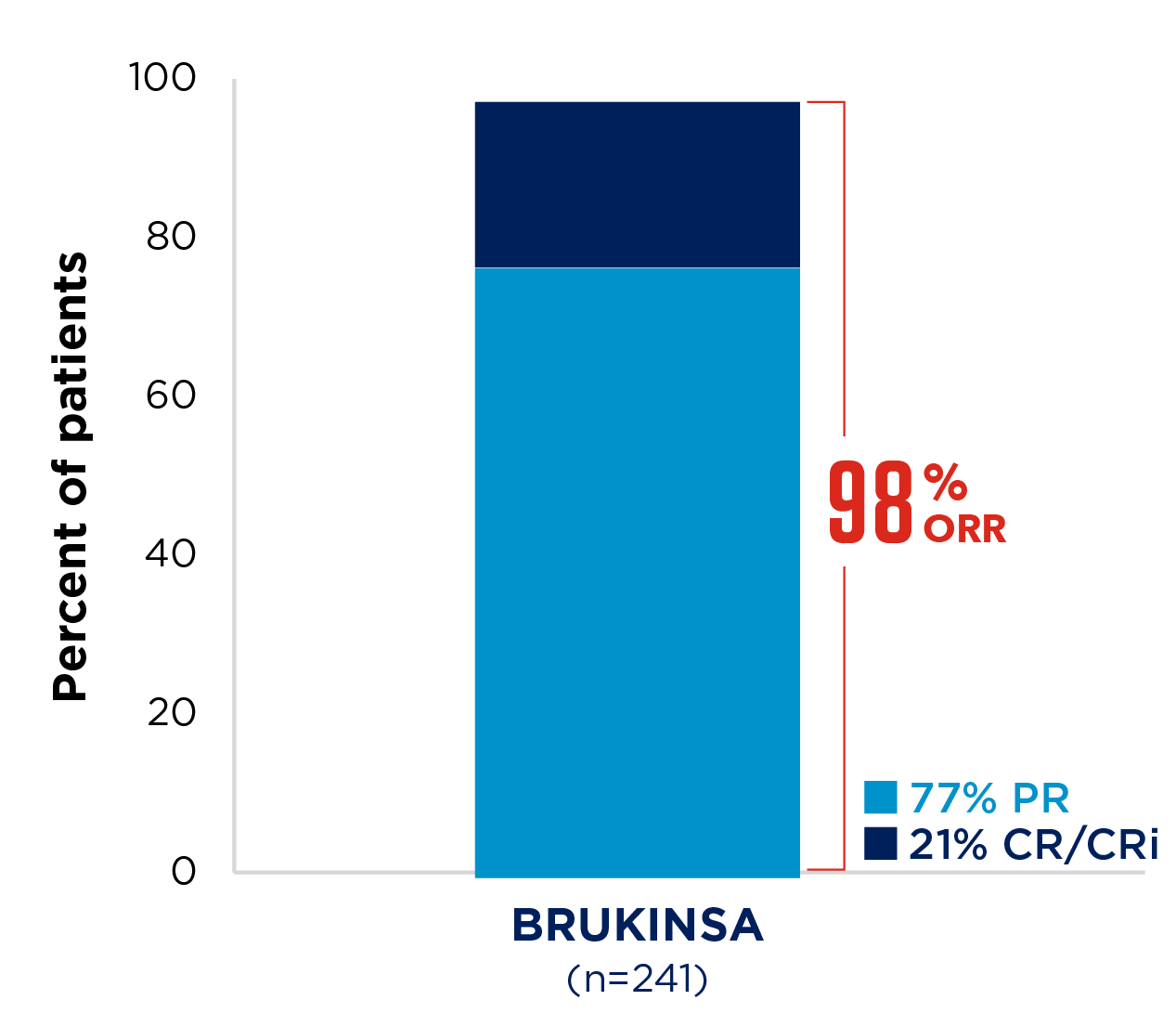
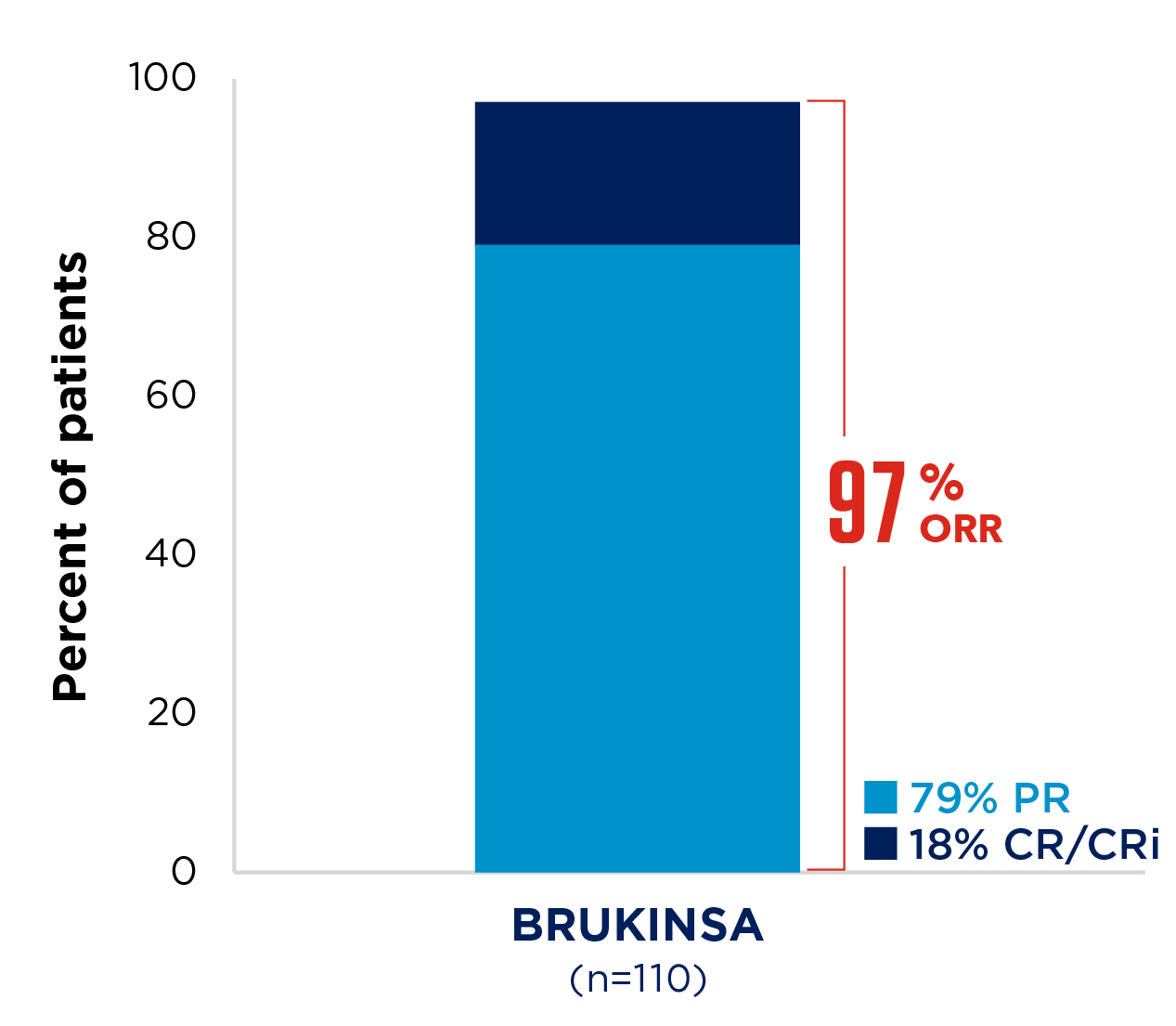
SEQUOIA was a randomised, open-label, Phase 3 trial of BRUKINSA vs BR in patients with TN CLL. Because patients with CLL/SLL whose tumours exhibit del(17p) have an unfavourable prognosis and respond poorly to standard CIT, patients with del(17p) mutations were assigned to receive BRUKINSA in a separate single-arm exploratory analysis. Cohort 1 enrolled patients without del(17p): BRUKINSA vs BR (n=479). Cohort 2 enrolled patients with del(17p); BRUKINSA single arm (n=111).5
ALPINE was an open-label, randomised, Phase 3, multicentre trial in patients with R/R CLL who received ≥1 prior systemic therapy.7
*The ELEVATE-RR study demonstrated noninferior PFS with acalabrutinib vs ibrutinib.8
AE, adverse event; BR, bendamustine + rituximab; BTK, Bruton’s tyrosine kinase; CI, confidence interval; CIT, chemo-immunotherapy; CLL, chronic lymphocytic leukaemia; CR, complete response including complete response with incomplete bone marrow recovery; GHS, global health status; GI, gastrointestinal; HR, hazard ratio; IRC, independent review committee; MCL, mantle cell lymphoma; mPFS, median progression-free survival; MZL, marginal zone lymphoma; NICE, National Institute for Health and Care Excellence; NE, not estimable; ORR, overall response rate; PAS, NHSScotland patient access scheme; PFS, progression-free survival; PR, partial response including nodular partial response; PR-L, partial response with lymphocytosis; QoL, quality of life; R/R, relapsed or refractory; SLL, small lymphocytic lymphoma; SMC, Scottish Medicines Consortium; TN, treatment-naïve; WM, Waldenström’s macroglobulinaemia.