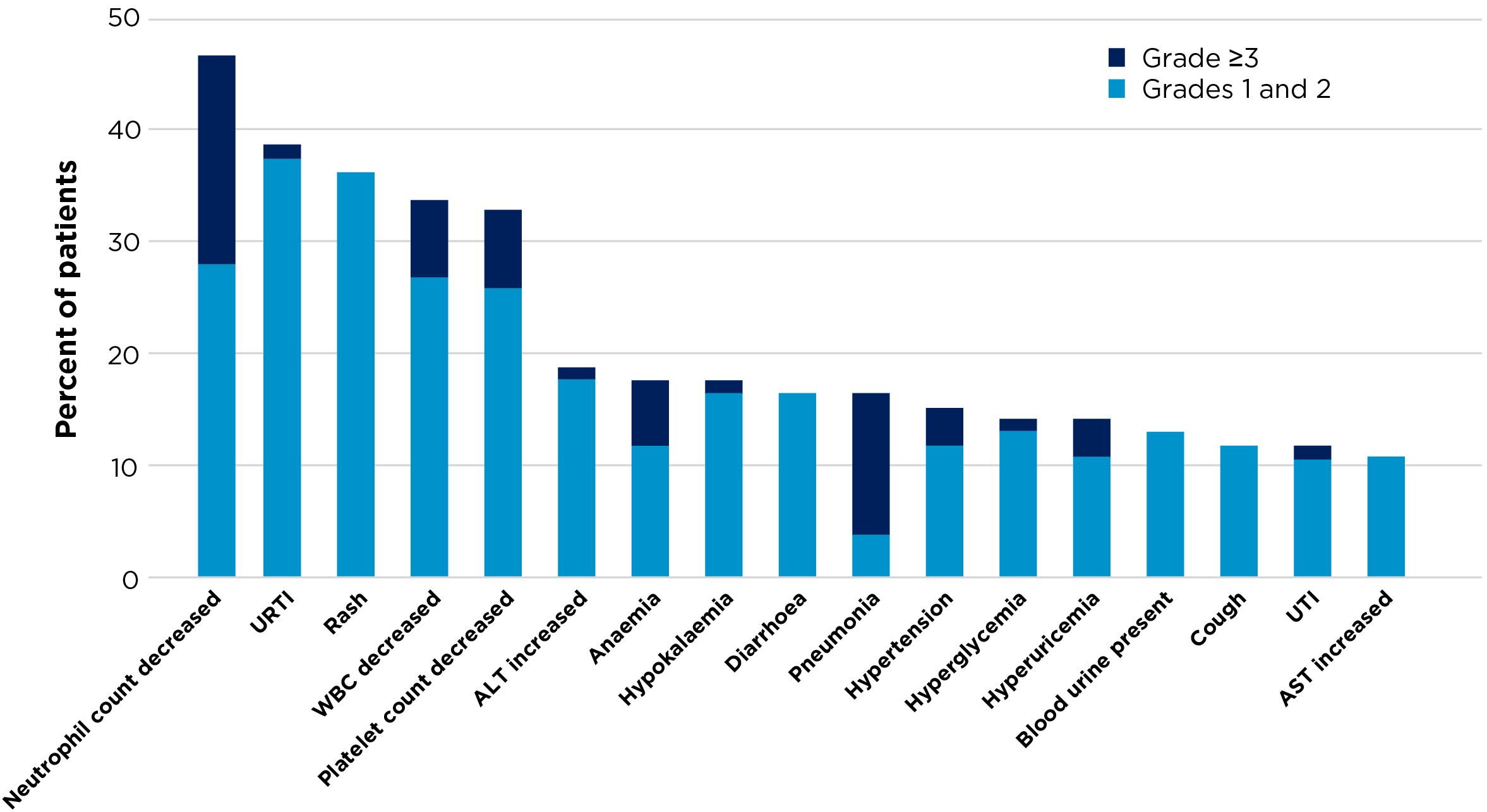
Study 206 (NCT03206970) is a Phase 2, single-arm, open-label study that investigated the efficacy and safety of BRUKINSA in patients with relapsed or refractory mantle cell lymphoma in China.3

This site is intended for UK healthcare professionals only. If you are not a UK healthcare professional,
please visit our dedicated site for more information at beonemedicines.co.uk
ONE BTK INHIBITOR: MANY POSSIBILITIES1,2
BRUKINSA as monotherapy is indicated for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.1
Study 206 (NCT03206970) is a Phase 2, single-arm, open-label study that investigated the efficacy and safety of BRUKINSA in patients with relapsed or refractory mantle cell lymphoma in China.3


At 18.4 months median follow-up, the IRC-assessed overall response rate was 84% (95% CI: 74.2, 90.8%), with 69% of patients achieving a complete response3
Median follow-up: 18.4 months
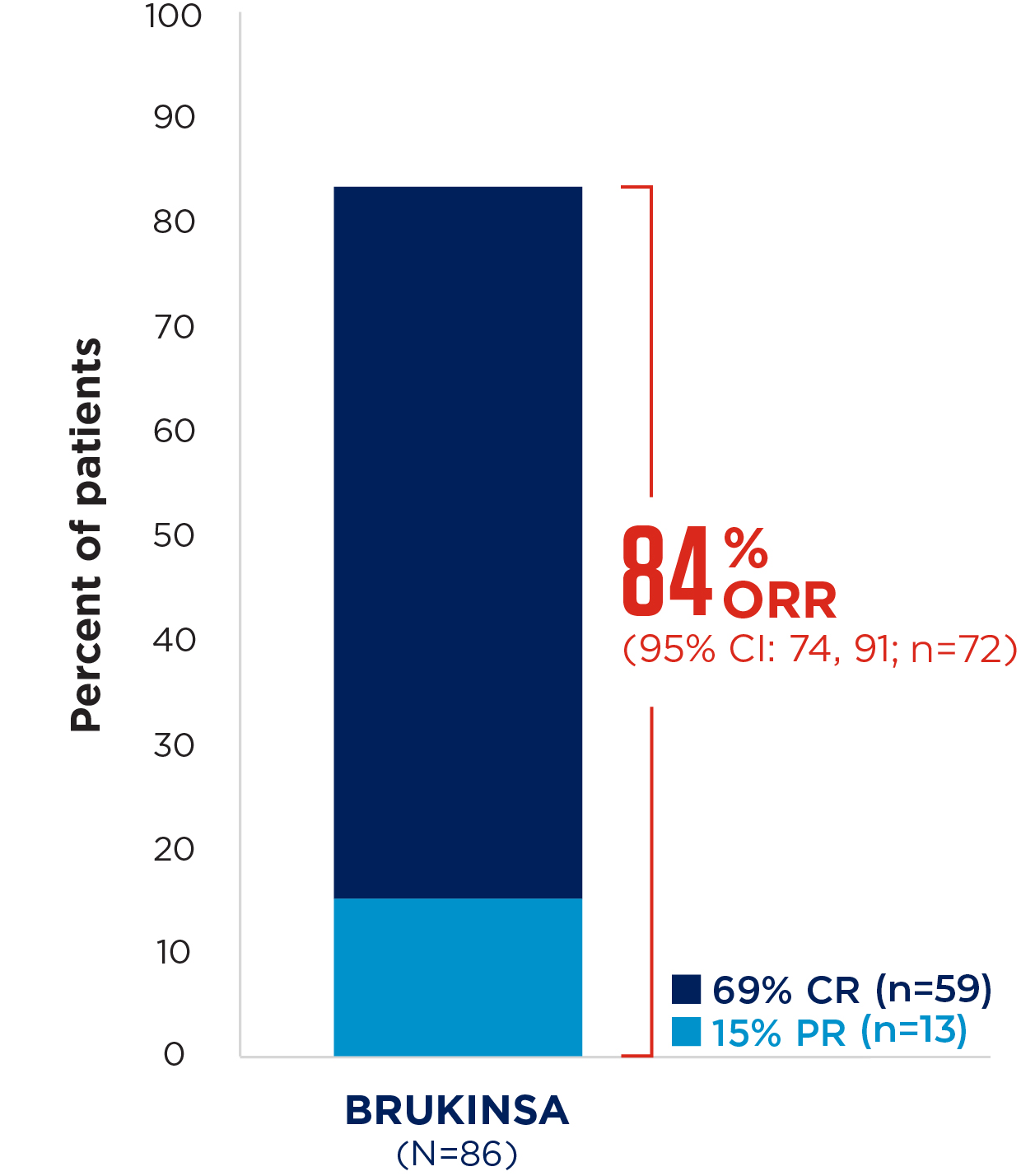
Figure adapted from Song Y, et al. 2020.3
Median follow-up: 33.3 months
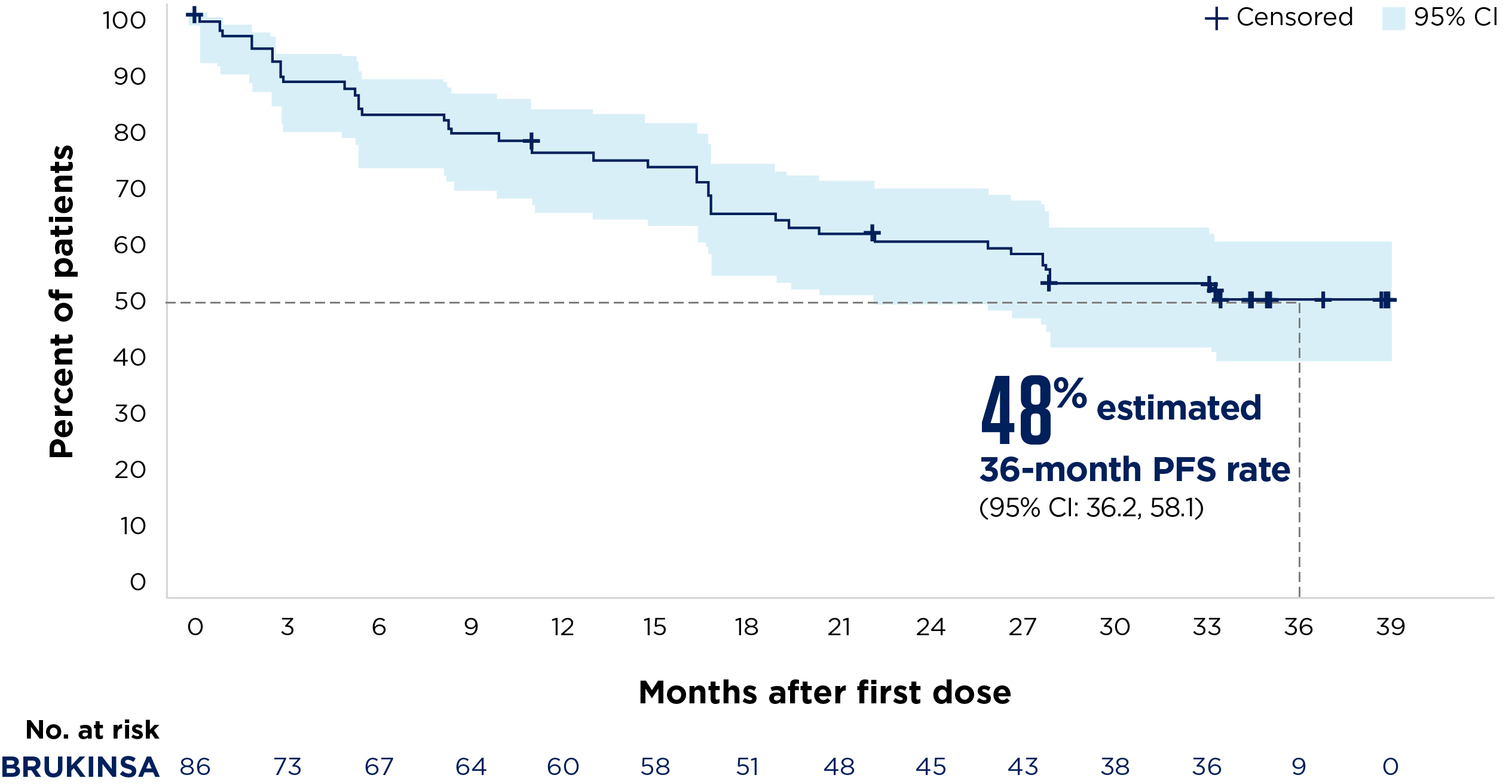
Figure adapted from Song Y, et al. 2022.4

Adapted from Song Y, et al. 20224

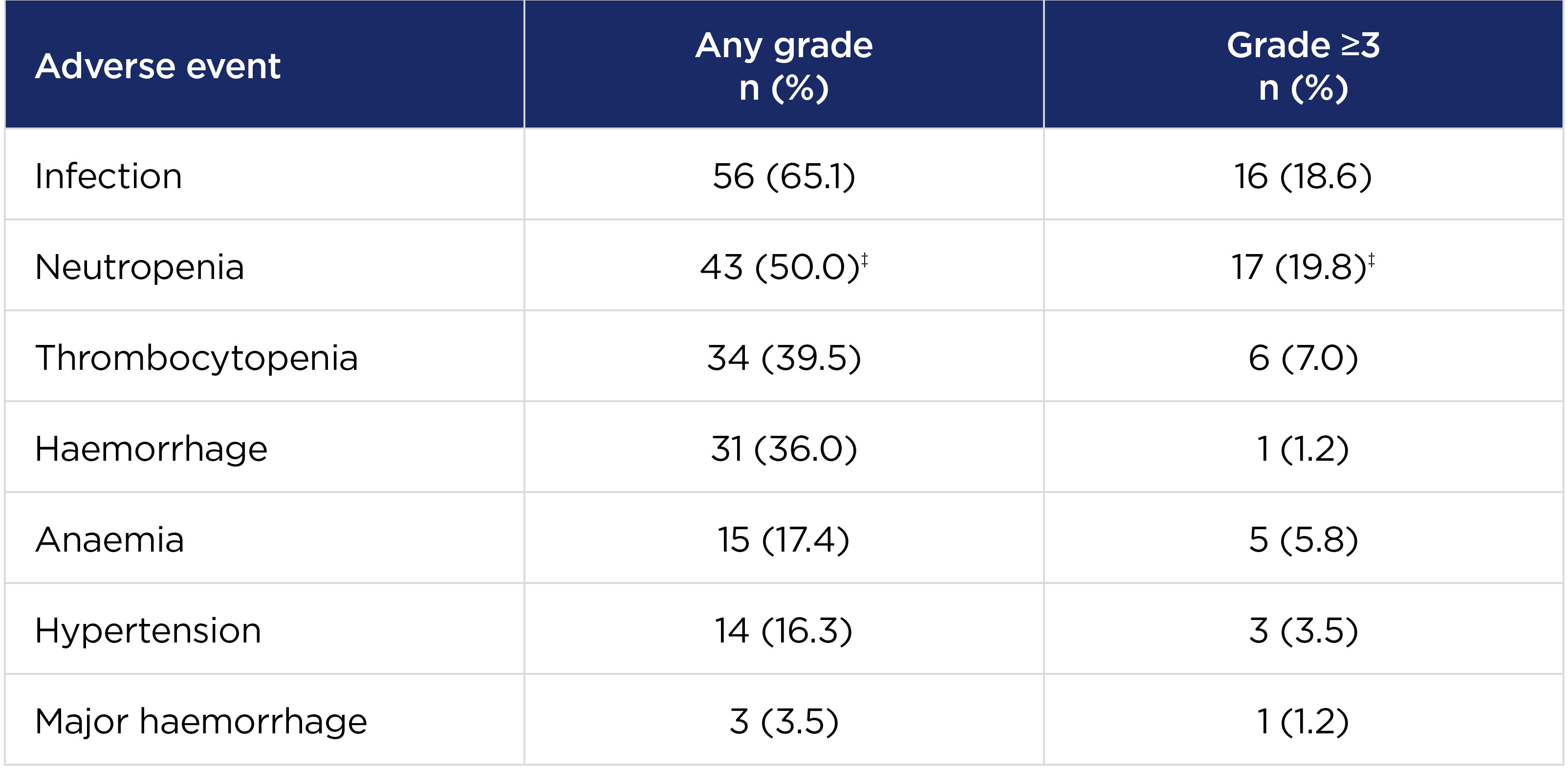
Median treatment duration 27.6 months, N=864