The MAGNOLIA study (NCT03846427) is a global, Phase 2, single-arm, open-label study that investigated the efficacy and safety of BRUKINSA in patients with relapsed or refractory marginal zone lymphoma.3


This site is intended for UK healthcare professionals only. If you are not a UK healthcare professional,
please visit our dedicated site for more information at beonemedicines.co.uk
ONE BTK INHIBITOR: MANY POSSIBILITIES1,2
BRUKINSA as monotherapy is indicated for the treatment of adult patients with marginal zone lymphoma (MZL) who have received at least one prior anti-CD20-based therapy.1
The MAGNOLIA study (NCT03846427) is a global, Phase 2, single-arm, open-label study that investigated the efficacy and safety of BRUKINSA in patients with relapsed or refractory marginal zone lymphoma.3


After 28 months of follow-up, the overall response rate was 68% (95% CI: 55.6, 79.1), with 26% of patients achieving a complete response4

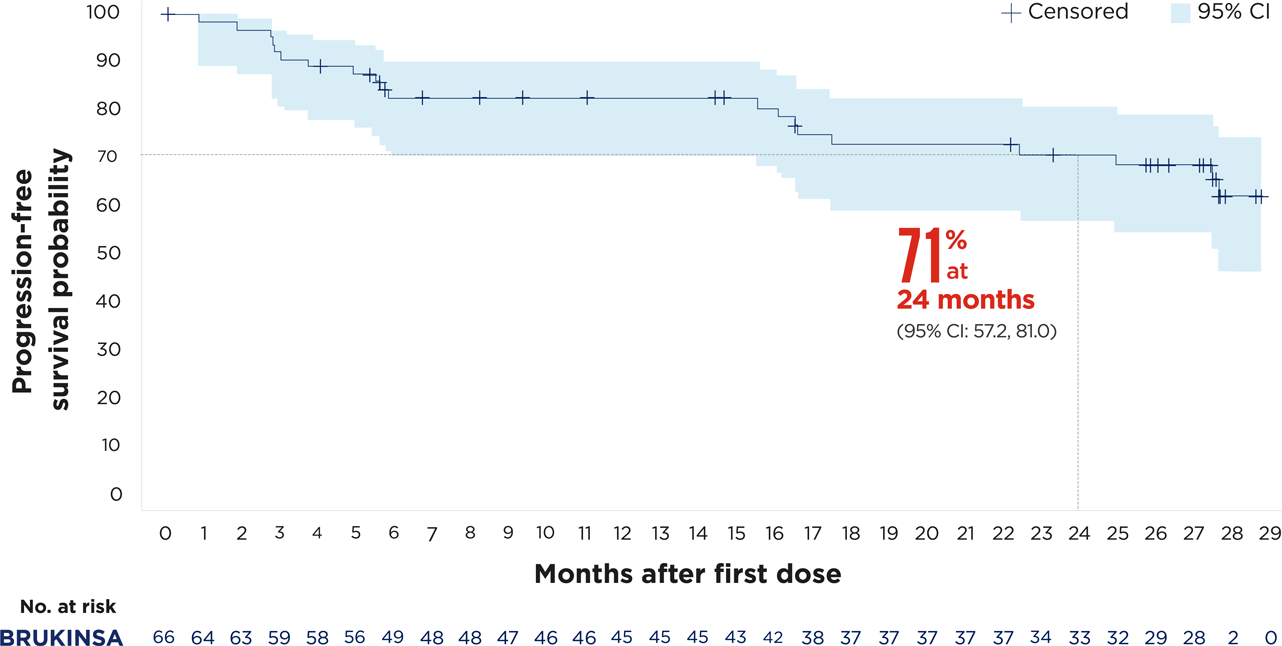
Figures adapted from Opat S, et al. 2023.4

Summary of AEs that occurred during treatment4
| Event | n (%) (N=68) |
|---|---|
| ≥1 AE | 68 (100) |
| Grade≥3 AEs | 33 (48.5) |
| Serious AEs | 30 (44.1) |
| AE leading to dose interruption | 25 (36.8) |
| AE leading to dose reduction | 0 (0) |
| AE leading to discontinuation | 5 (7.4) |
| AE leading to death | 5 (7.4) |

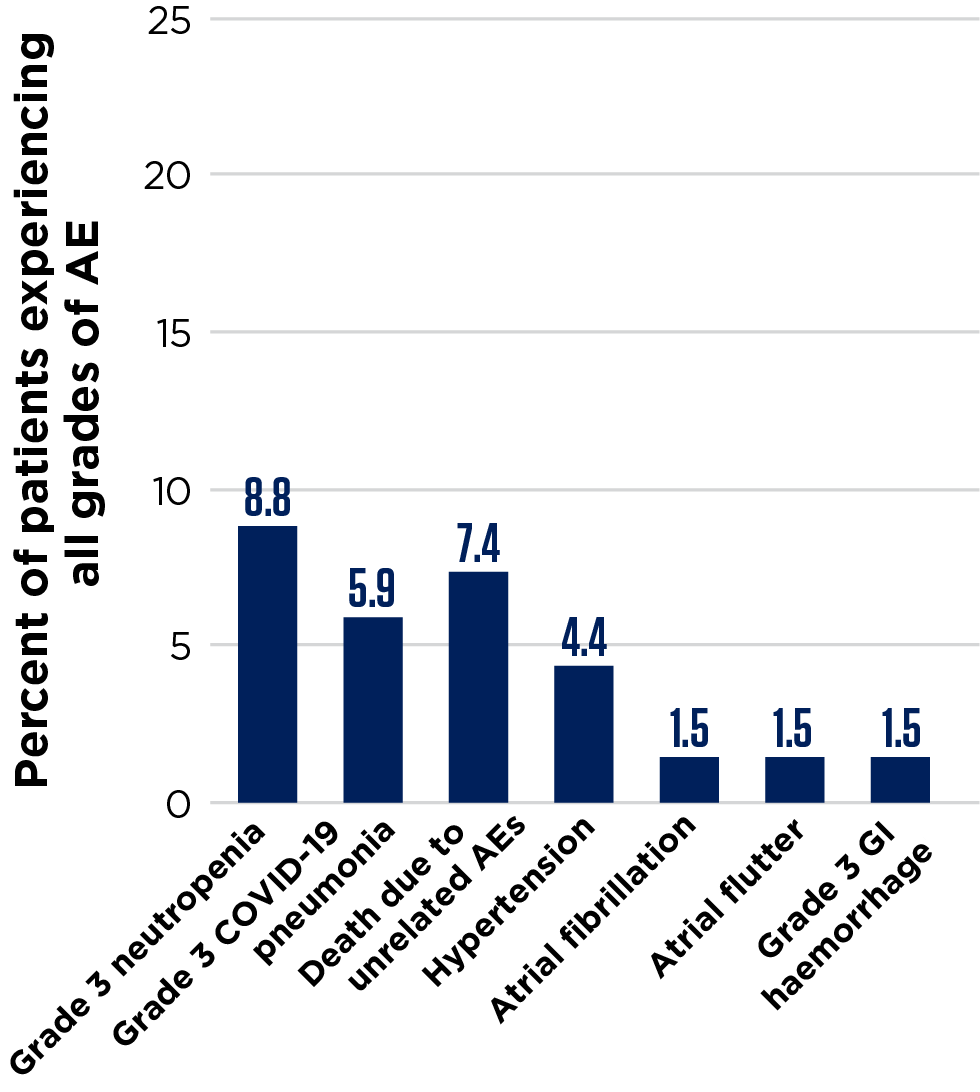
Figures adapted from Opat S, et al. 2023.4